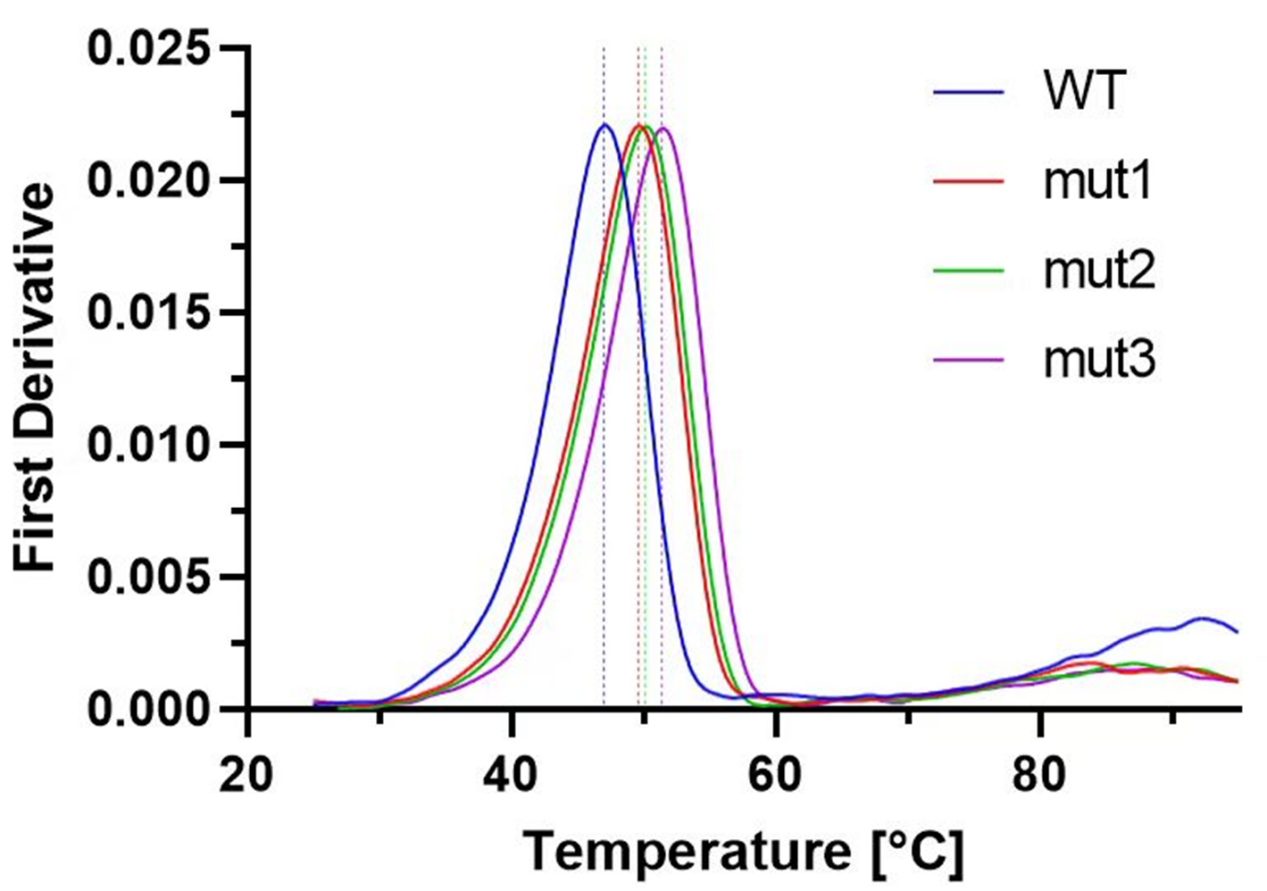
To compare the changes in thermal stability of wild-type protein D and mutant protein.
Technical difficulties:
▇The traditional dye-based DSF method resulted in unclear differences and poor peak quality in the spectra of the
wild-type and mutant proteins.
Solution:
▇The label-free Nano-DSF, based on the intrinsic tryptophan fluorescence shift during protein unfolding, quickly provided
high-quality results.
▇The spectra intuitively displayed the relative thermal stability of each protein.
Results: