-
+86 18962587269
-
contact@readcrystal.com
-
Changshu High-tech Industrial Development Zone, Suzhou, Jiangsu Province

Introduction
Fifty years after Werner's foundational work in coordination chemistry, the discovery of solvent-containing crystals led to the emergence of porous structures for gas storage. While earlier examples lacked stability, MOF-2 and MOF-5 marked a pivotal moment, enabling the synthesis of porous structures with tunable compositions through strong organic-inorganic bonds. MOFs and COFs, due to their structural versatility, have surged in significance across various fields like photocatalysis, gas storage, and energy applications.
Challenges in Structural Analysis
Structural analysis profoundly influences material properties. Presently, single crystal X-ray diffraction remains the primary method for MOF and COF structural analysis. Yet, obtaining high-quality single crystals is laborious and time-consuming. Many attempts fall short in producing crystals sizable enough for accurate X-ray diffraction.
MOFs Analysis
MOFs' single crystal synthesis demands intensive exploration but falls short in acquiring adequate crystals for analysis. This bottleneck impedes their widespread study and application.
COFs Analysis
Conjugated organic frameworks (COFs) are a type of crystalline, porous materials composed of interconnected organic units held together by covalent bonds. They have strong design ability. Researchers use diverse organic synthesis techniques to design and synthesize components while employing framework chemistry to construct and functionalize the skeleton of COFs. In addition, COFs, as a porous structure composed of lightweight elements such as C, H, and N, have a low density (as low as 0.13 g/cm 3 ) and a high specific surface area (up to 5083 cm 2 /g). Since the Yaghi research group reported the first COF in 2005, these materials have shown broad application prospects in the fields of molecular adsorption and separation, catalysis, optoelectronics, sensing, and energy applications. Their results have yielded significant advancements in research across these fields.
While Ma Tianqiong succeeded in synthesizing the initial single crystal of COFs and provided an effective strategy for their synthesis, this method was time-consuming. Specific adjustments need to be tested for different COFs to improve crystallinity. At present, most COFs are synthesized without this technique, relying instead on analyzing COF powder to determine their structure. Due to the large unit cell parameters of COFs, it is challenging to solve the structure by powder X-ray diffraction alone. Many COFs, especially two-dimensional COFs, due to poor crystallinity, can only obtain the unit cell parameters based on powder diffraction patterns. To compensate, researchers construct artificial structural models and conduct simulations based on these models. By comparing simulated results—such as hole diameter and specific surface area—to experimental values, they select a structural model that best matches the data, presuming it to represent the genuine COF structure. Refining powder X-ray diffraction patterns solely refines the peak shape (Pawley or Lebail refinement), without directly refining the structure itself. As understanding deepens, it is found that the structure obtained through this approach can sometimes be inaccurate.
Introducing MicroED
In MOFs/COF research, the most time-consuming step is to determine the structure of the product. However, using MicroED (Microcrystalline Electron Diffraction) for structural analysis, this might no longer be true. Today, researchers can efficiently collect data and analyze the structure of nanometer-sized single crystal samples. This technique significantly reduced the challenge of cultivating nanometer-sized crystals, thereby compressing the single crystal cultivation cycle from weeks to one day or even hours, Especially for for the more challenging MOFs. Moreover, It’s proving invaluable for COFs, which are more difficult in single crystal cultivation. MicroED has unveiled the accurate structures of numerous previously unknown COFs. Its potential to revolutionize fields like organic chemistry earned MicroED a spot among Science journal's top ten technological breakthroughs in 2018.
MicroED is a dynamic three-dimensional electron diffraction technique. It continuously records different patterns at various angles using the TEM goniometer. The sample rotates along the axis of the goniometer through the data collection process, which typically lasts only a few minutes for a single sample. This swift collection process makes it suitable for handling electron beam-sensitive samples. One of MicroED's key advantages stems from the powerful interaction between electrons and substances, which is stronger than that of X-rays. Electron diffraction requires significantly smaller sample sizes—about 2-3 orders of magnitude smaller than those needed for X-rays. It allows for the examination of crystals as small as 100 nanometers. Moreover, the three-dimensional electron diffraction method also reduces the dynamic effects. This allows the analysis and refinement of structure information through kinematic methods.
Below, we introduce several examples of structural analysis of MOFs and COFs through MicroED.
Case 1
Sun Junliang's group at Peking University, and his collaborators, used cRED (comparable to MicroED ) technology to collect electron diffraction data of a 5-10 μm-sized two-dimensional conductive MOF named Cu3HHTT2. They obtained a resolution of 1.5 Å (Figure 1). Based in this data, the structure of the MOF was analyzed, revealing a rare perfect AA stack. The distance between adjacent layers in this MOF is shorter than any other known two-dimensional MOFs. The accuracy of this structure was verified through N2 adsorption experiments and high-resolution electron microscope photos.
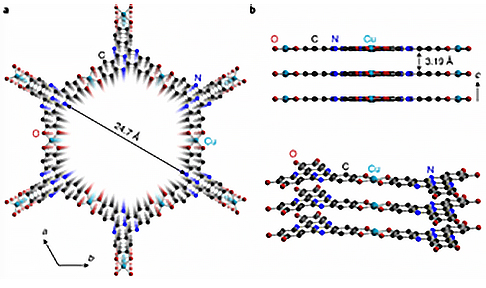
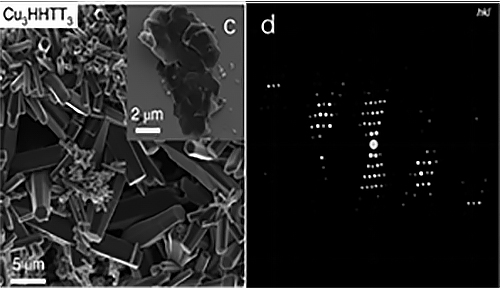
Figure 1 (a) and (b) Structural diagram of Cu3HHTT2, (c) and (d) Morphology and three-dimensional reciprocal lattice of Cu3HHTT2, respectively.
Case 2
Xiaodong Zou’s group at Stockholm University, and his collaborators, analyzed the structure of photochemically active MOFs PCN-415 and PCN-416. Due to their incredibly small crystal size—merely 500 nm (Figure 2), the team employed MicroED technology to obtain the structures of these MOFs. The acquired structures were further confirmed by Rietveld refinement using powder X-ray diffraction data.
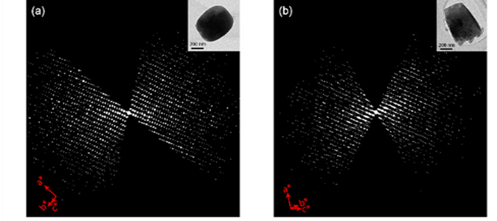
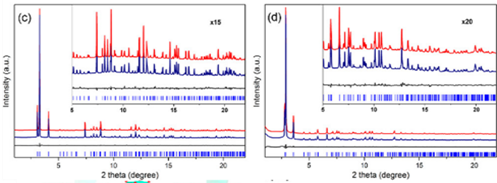
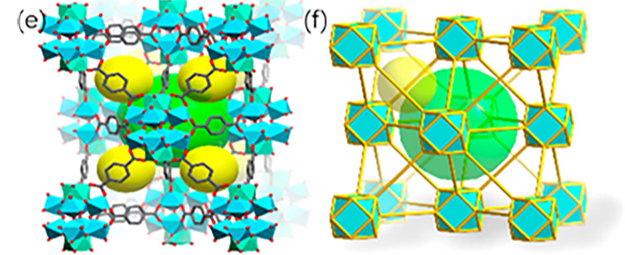
Figure 2 (a) and (b) Three-dimensional reciprocal lattice reconstructed from cRED data of PCN-415 and PCN-416; (c) and (d) Rietveld refinement of PXRD of PCN-415 and PCN-416; (e) Schematic structural diagram of PCN-415; (f) Schematic diagram of fcu topology of PCN-415
Case 3
Xiaodon Zou's research group also synthesized a novel porous cobalt MOF, Co-CAU-36, sized at approximately 500 nm (Figure 3). Through MicroED at a temperature below 100K, they captured eight sets of high-resolution images. Based on the three-dimensional electron diffraction data, the positions of all metal ions and connectors except hydrogen atoms were obtained. Through refinement techniques, the position of the solvent was also identified, marking the pioneering use of electron diffraction for solvent determination and refinement.
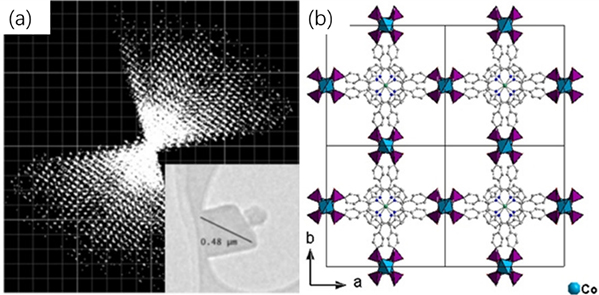
Figure 3 Schematic diagram of (a) three-dimensional reciprocal lattice (b) structure of Co-CAU-36
Case 4
Sun Junliang’s research group at Peking University and Wang Cheng’s research group at Wuhan University collaborated to obtain the structure of the 3D-TPB series COF through MicroED (Figure 4-5). The resolution of the data is 0.9-1.0 Å. Their collaborative effort demonstrates the groundbreaking capability of three-dimensional electron diffraction technology to precisely locate all non-hydrogen atoms within the structure.
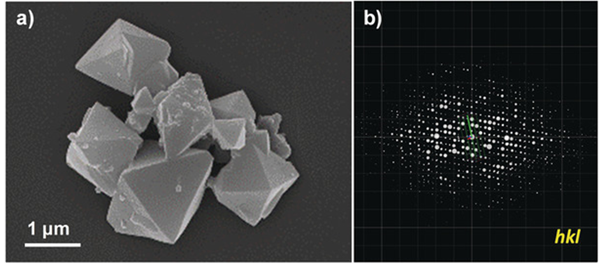
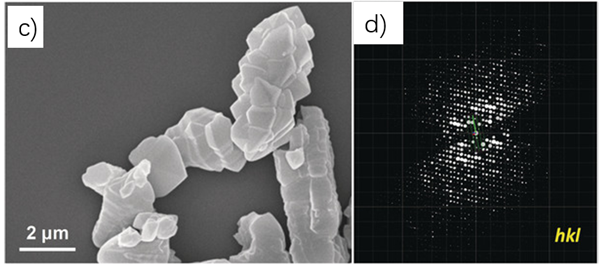
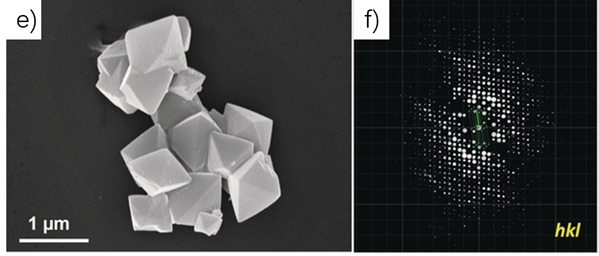
Figure 4 Morphology of 3D-TPB-COF and its three-dimensional reciprocal lattice
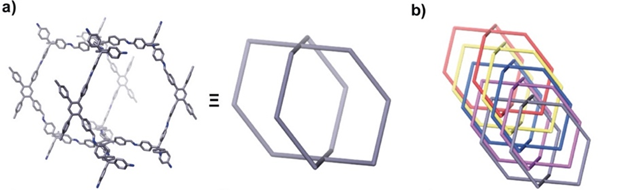
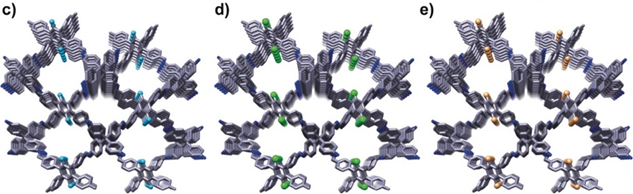
Figure 5 Structural diagram of 3D-TPB-COF
Summarize
Single crystal X-ray diffraction is the main method for structural analysis of MOFs and COFs. However, it requires large-single crystal samples. MicroED technology demands only nanoscale or powder crystals for analysis, which greatly reduces the difficulty of crystal cultivation. Many unknown structures in MOFs and COFs have been analyzed through MicroED. ReadCrystal specializes in MicroED technology. Our internationally leading MicroED platform is renowned for its efficiency in rapidly and precisely analyze the structure of nanocrystals. and obtain structural information without the need for a long single crystal cultivation process, which provides scientific research and academic results. Provide technical support. In addition, MicroED can further test materials such as proteins, peptides, and small drug molecules.
References:
1. Yaghi , OM Reticular chemistry: molecular precision in infinite 2D and 3D. Molecular Frontiers Journal 3, 66-83 (2019).
2. Kitagawa, S. Metal–organic frameworks (MOFs). Chemical Society Reviews 43, 5415-5418 (2014).
3. Yaghi , OM, Kalmutzki , MJ & Diercks , C. Introduction to reticular chemistry. Mol. Front. J. 4 (2019).
4. Dou, J.-H. et al. Atomically precise single-crystal structures of electrically conducting 2D metal–organic frameworks. Nature Materials 20, 222-228 (2021).
5. Yuan, S. et al. [Ti8Zr2O12 (COO) 16] cluster: An ideal inorganic building unit for photoactive metal–organic frameworks. ACS central science 4, 105-111 (2018).
6. Wang, B. et al. A Porous Cobalt Tetraphosphonate Metal–Organic Framework: Accurate Structure and Guest Molecule Location Determined by Continuous‐Rotation Electron Diffraction. Chemistry–A European Journal 24, 17429-17433 (2018).
7. Gemmi , M. et al. 3D electron diffraction: the nanocrystallography revolution. ACS central science 5, 1315-1329 (2019).
8. Diercks , CS & Yaghi , OM The atom, the molecule, and the covalent organic framework. Science 355 (2017).
9. Baldwin, LA, Crowe, JW, Pyles , DA & McGrier , PL Metalation of a mesoporous three-dimensional covalent organic framework. Journal of the American Chemical Society 138, 15134-15137 (2016).
10. Cote, AP et al. Porous, crystalline, covalent organic frameworks. science 310, 1166-1170 (2005).
11. Ma, T. et al. Single-crystal x-ray diffraction structures of covalent organic frameworks. Science 361, 48-52 (2018).
12. Gao, C. et al. Isostructural Three-Dimensional Covalent Organic Frameworks. Angewandte Chemie International Edition 58, 9770-9775 (2019).
+86 18962587269
contact@readcrystal.com
Changshu High-tech Industrial Development Zone, Suzhou, Jiangsu Province