-
+86 18962587269
-
contact@readcrystal.com
-
Changshu High-tech Industrial Development Zone, Suzhou, Jiangsu Province

Structural biology is an interdisciplinary field that combines molecular biology, biochemistry, and biophysics. The research objects are biological macromolecules, such as proteins and nucleic acids. The purpose is to use biological or physical methods to reveal the structures of these molecules, elucidate their functional mechanisms, and offer a better understanding of life activities. Moreover, given that many drug molecules primarily interact with proteins, structural biology plays an important role in guiding drug research and development. It seamlessly connects the dots from mechanisms to drug development.
The study of proteins in biological macromolecules relies on several main research methods, such as nuclear magnetic resonance (NMR), X-ray crystallography, and cryo-electron microscope (cryo-EM). NMR mainly targets sample types with very small molecular weight (about 20kDa) in solution. In recent years, new technologies have also emerged. Microelectron diffraction (MicroED) is a technology that uses cryo-electron microscopy to analyze the structure of tiny crystals. Unlike X-rays, electron beams interact more intensely with substances, enabling the analysis of challenging problems in X-ray crystallography and facilitating the examination of processed nanocrystal structures. MicroED provides especially valuable for protein samples historically difficult to crystallize. This innovative approach fills critical gaps in existing technologies, providing structural biologists with a promising new tool. This technology was named one of the top ten breakthrough technologies in 2018 by Science Journal.
● X-ray diffraction: the traditional structural analysis method
As early as 1895, WC Roentgen discovered X-rays. The discovery of X-rays promoted the development of modern biology, and it had a profound impact on the scientific and technological landscape. In 1912, MV Laue, W. Friedrich, and Roentgen's student P. Knipping conducted diffraction experiments on copper sulfate crystals and obtained negative results marked by thick, oval spots in X-ray crystal diffraction. Their study expanded to ZnS, PbS, and NaCl, yielding clear and symmetrical four-fold diffraction patterns. Laue also proposed the Laue equation to describe X-ray diffraction in crystals. Shortly after the X-ray diffraction photos of ZnS crystals, WL Bragg repeated the experiment and derived the Bragg equation in 1913. These discoveries marked the birth of X-ray crystallography. By 1957, X-ray crystallography had solved the structure of the first biological macromolecule, sperm whale myoglobin. So far, X-ray crystallography has unraveled the structures of approximately 150,000 biological macromolecules. The rapid evolution in three-dimensional protein structure analysis stems from its unique advantages: its capability to achieve near-atomic resolution and its versatility, transcending limitations regarding the size and composition of proteins. Whether dealing with a multi-subunit complex or a small molecular weight peptide, this method delivers optimal results. However, this method relies on high-quality, sizable crystals forms both its advantages and limitations. Obtaining such crystals is challenging for objects with large molecular weights or flexible structures.
● Single particle analysis technology based on cryo-electron microscopy
Cryo-electron microscopy has developed rapidly and gradually profoundly impacting structural biology. The technique traces back to the 1930s when Siemens built the first commercial electron microscope. Early methods used heavy metal staining or sugar embedding that damaged sample processing and observations conducted under room temperature conditions. By J. Dubochet et al. in 1984, rapid freezing technology or cryo-freezing emerged. This technique involved rapidly immersing biological samples into liquid ethane at a temperature cooled to liquid nitroge1 , 2 . This process yielded well-preserved frozen samples with a suitable ice thickness, allowing for the capture of thousands of images of uniformly dispersed biological macromolecule particles within the ice layer. These two-dimensional photos underwent analysis through algorithms to finally obtain their structures, a method referred to as single-particle analysis technology. In recent years, the emergence of direct electron detection cameras (direct detection devices, DDD) and the development of data processing software have significantly increased the number of resolved three-dimensional structures of biological macromolecules (Figure 1). For example, a study published in Science in March 2020, using cryo-electron microscopy single-particle analysis to determine the structure of human pancreatic protein binding to glucagon and different types of heterotrimeric G proteins (G s or G i1) in the. Glucagon receptor (GCGR) at resolution of 3.7 Å and 3.9 Å (Figure 2). The combination of structural and pharmacological data from this study provides important insights into the treatment of type II diabetes and obesity 3.
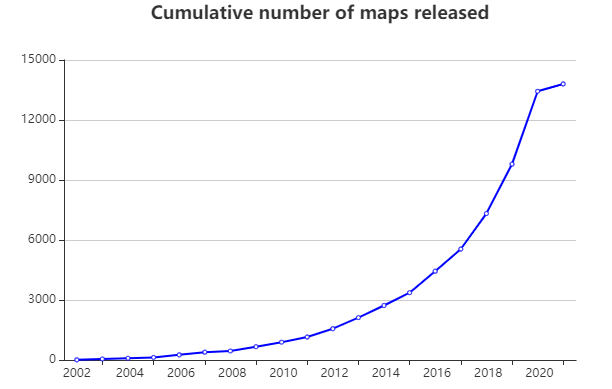
Figure 1. Evolution of Cryo-Electron Microscopy Analysis: Shifting Trends in Biological Macromolecule Structures.
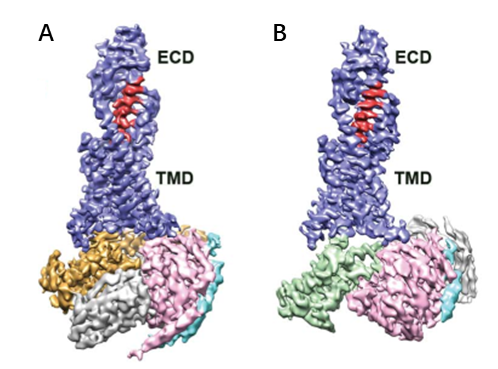
Figure 2. Cryo-electron microscopy structures of Glucagon-GCGR-GS -Nb35 and glucagon-GCGR-Gi1 -Scfv16.
Single-particle analysis technology represents a breakthrough in studying complex structures like membrane proteins and macromolecular complexes without relying on crystallization. It offers an advantage by requiring minimal sample quantities. However, achieving sample homogeneity remains a significant challenge despite the reduced sample needs. Improvements in detectors and algorithms have advanced this technology to achieve resolutions nearing 2 Å4, although such high resolution remains relatively uncommon. Typically, structures are resolved around 3 Å. Nevertheless, the essence of human civilization lies in our exploration of mysterious nature and scientific truth. The continuous development and advancement of various research fields will inevitably influence and promote each other.
● MicroED: Uniting Crystallography and Cryo-Electron Microscopy
In the early days of electron microscope development, X-ray crystallography flourished, with diffraction theory and data processing methods gradually maturing. The combination of electron microscopy and crystallography generated electron crystallography. In 2007, scientists at Stockholm University cleverly combined crystallography and electron microscopy used cryo-electron microscopy to visualize crystals, and developed an important new technology - MicroED (Micro Electron Diffraction). MicroED uses electrons to diffract tiny crystals, collecting and analyzing electron diffraction data to elucidate protein structures. Electrons, due to their shorter wavelength, exhibit strong material scattering (10,000 times stronger than X-rays), yielding higher achievable resolutions. Moreover, this method requires tiny crystal sizes, enabling submicron-sized crystals to produce robust diffraction signals, offering a breakthrough for samples where crystal enlargement isn’t feasible. Recognized as one of the top ten technological breakthroughs in 2018 by Science jpurnal, MicroED initally solved model samples such as lysozyme, catalase, and Ca 2+ -ATPase at atomic resolution. With further development, it is no longer limited to model samples. It has also analyzed the structures of unknown proteins such as α-synuclein central peptide and R2lox enzyme (Figure 3), achieving resolution as high as ~1 Å. After 2016, MicroED developed rapidly, unraveling high-resolution protein structures (Figure 4), including prions and FUSLC (fused in sarcoma low-complexity domain), obtaining a high resolution of about 1 Å (Figure 4). Then, MicroED was applied on more challenging targets, including membrane proteins such as complexes and ion channels (Figure 4). Starting in 2018, MicroED has been combined with cryo-Focused Ion Beam (cryo-FIB) technology to thin slightly larger crystals, which previously unsuitable for X-ray crystallography but now compatible with MicroED. This expansion broadened the crystal size range to tens of microns, filling the gap between MicroED and X-ray crystallography.
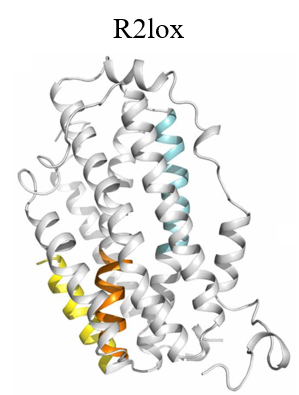
Figure 3. R2lox structure analyzed by MicroED
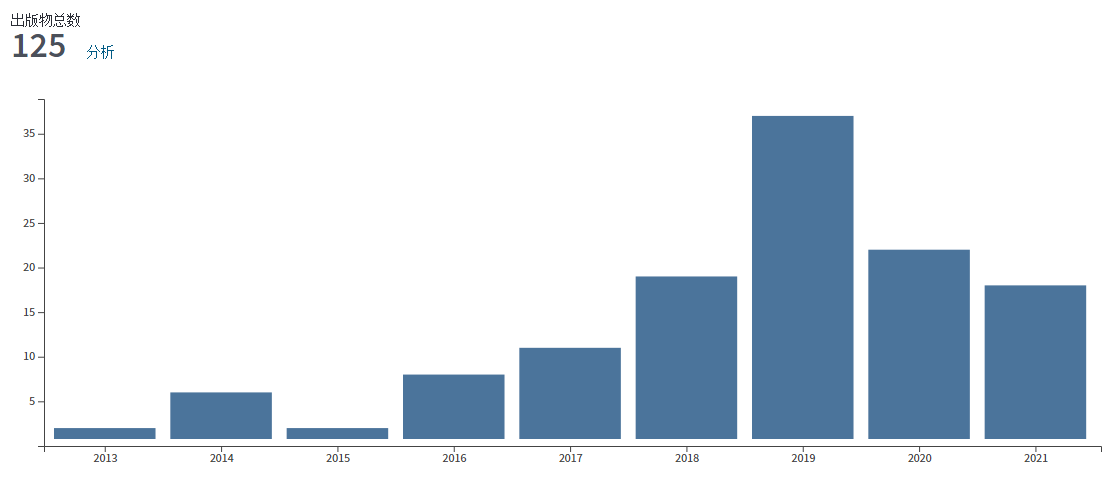
Figure 4. MicroED research statistics
● Simple process, high resolution, short time - MicroED opens up a new way of structural biology exploration
Similar to X-ray crystallography, MicroED capitalizes on electron diffraction, mirroring the behavior of X-rays on crystalline samples. The process involves positioning the object plane of the intermediate mirror at the back focal plane of the objective lens. This setup amplifies the electron diffraction spectrum, projecting it onto a fluorescent screen as a diffraction pattern within the transmission electron microscope. The practical application of MicroED within a transmission electron microscope involves pre-set configuration for the intermediate mirror in imaging and diffraction modes. The two modes can be switched by pressing the “Diffraction button”. MicroED is an invaluable asset in structural biology research.
The unique advantages of MicroED extend beyond biological macromolecules to small chemical molecules, natural products, and pharmaceutical preparations. The suitability of this technology for powder-form chemical molecules aligns perfectly with its research scope, enabling the extraction of structural information within a remarkably short timeframe, typically within hours. Numerous successful cases using MicroED to study small chemical molecules have verified its efficiency and applicability (Figure 6). The application of MicroED in both biological macromolecules and small molecule drugs demonstrates its great application potential in structural biology, providing structural biologists with a promising powerful tool.
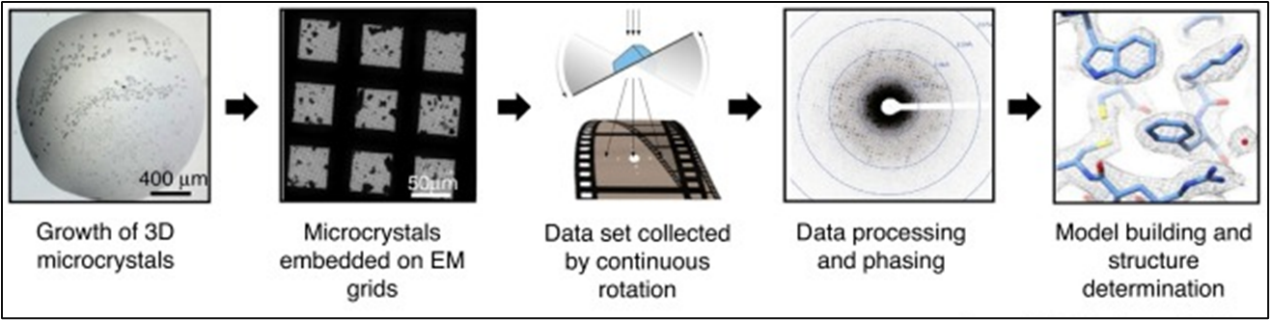
Figure 5. MicroED Workflow
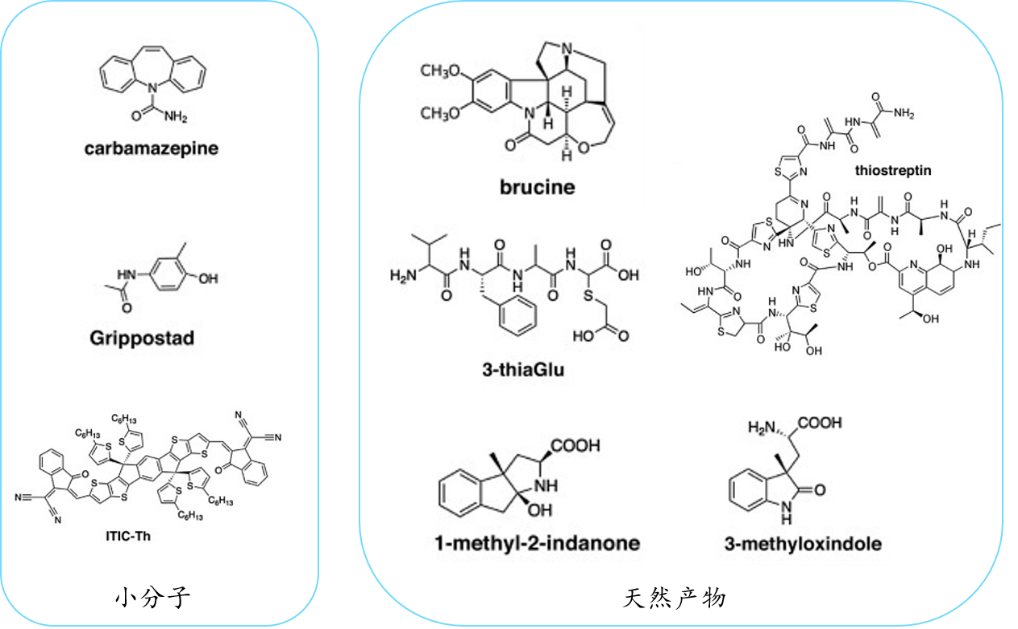
Figure 6. Examples of small chemical molecules and natural products obtained by MicroED
Generally speaking, whether it is X-ray crystallography, cryo-electron microscopy single-particle technology, or the emerging MicroED technology, scientists are gradually advancing through continuous attempts and optimization. They complement and promote each other in terms of technology and research scope, driving humanity closer to the truth of nature, and continuing to lead the development of life sciences. Regarding commercial structural biology testing services, ReadCrystal stands out with our advanced MicroED testing platform offering SCXRD and CryoEM -SPA testing services on an international scale.
Reference
1 Dubochet, J., Adrian, M., Lepault, J. & McDowall, A. W. Emerging techniques: Cryo-electron microscopy of vitrified biological specimens. Trends in Biochemical Sciences 10, 143-146 (1985).
2 Adrian, M., Dubochet, J., Lepault, J. & McDowall, A. W. Cryo-electron microscopy of viruses. Nature 308, 32-36 (1984).
3 Qiao, A. & Han, S. Structural basis of G(s) and G(i) recognition by the human glucagon receptor. 367, 1346-1352 (2020).
4 Yip, K. M. F., N. Atomic-resolution protein structure determination by cryo-EM. 587, 157-161 (2020).
5 Shi, D., Nannenga, B. L., Iadanza, M. G. & Gonen, T. Three-dimensional electron crystallography of protein microcrystals. Elife 2, e01345 (2013).
6 Xu, H. et al. Solving a new R2lox protein structure by microcrystal electron diffraction. Science Advances 5 (2019).
7 Nguyen, C. & Gonen, T. Beyond protein structure determination with MicroED. Current Opinion in Structural Biology 64, 51-58 (2020).
+86 18962587269
contact@readcrystal.com
Changshu High-tech Industrial Development Zone, Suzhou, Jiangsu Province